Register Today!
ISO 13485 Lead Implementor Brochure
Learning Objectives
- Acknowledge the correlation between ISO 13485 and other standards and regulatory frameworks
- Master the concepts, approaches, methods and techniques used for the implementation and effective management of a MDQMS
- Learn how to interpret the ISO 13485 requirements in the specific context of an organization
- Learn how to support an organization to effectively plan, implement, manage, monitor and maintain a MDQMS
- Acquire the expertise to advise an organization in implementing MDQMS best practices
Why should you attend?
ISO 13485 Lead Implementer training enables you to develop the necessary expertise to support an organization in establishing, implementing, managing and maintaining a MDQMS based on ISO 13485. During this training course, you will also gain a thorough understanding of the best practices of Medical Devices Quality Management Systems and be able to improve an organization`s overall performance by consistently providing safe and qualitative medical devices.
After mastering all the necessary concepts of Medical Devices Quality Management Systems, you can sit for the exam and apply for a “PECB Certified ISO 13485 Lead Implementer” credential. By holding a PECB Lead Implementer Certificate, you will be able to demonstrate that you have the practical knowledge and professional capabilities to implement ISO 13485 in an organization.
Who Should Attend?
- Managers or consultants involved in Medical Devices Quality Management
- Expert advisors seeking to master the implementation of a Medical Devices Quality Management System
- Individuals responsible for maintaining conformance with MDQMS requirements
- MDQMS team members
Course Overview
The “PECB Certified ISO 13485 Lead Implementer” exam fully meets the requirements of the PECB Examination and Certification Program. The exam covers the following competency domains:
- Fundamental principles and concepts of a MDQMS
- Planning a MDQMS implementation based on ISO 13485
- Implementing a MDQMS based on ISO 13485
- Performance evaluation, monitoring and measurement of a MDQMS based on ISO 13485
- Continual improvement of a MDQMS based on ISO 13485
- Preparing for a MDQMS certification audit
This course is expected to take approximately 5 days, including examination day, to complete.
Certification
After successfully completing the exam, you can apply for the credentials shown on the table below.
You will receive a certificate once you comply with all the requirements related to the selected credential.
For more information about ISO 13485 certifications and the PECB certification process, please refer to the
Certification Rules and Policies.
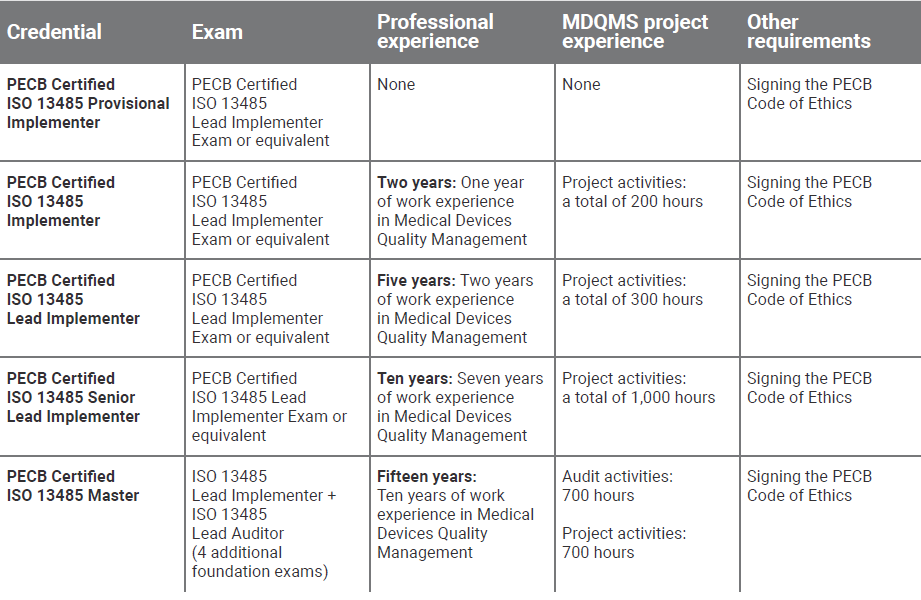
Note: PECB Certified Individuals who do possess the Lead Implementer and Lead Auditor Credentials are qualified for the respective PECB Master Credential, given they have taken 4 additional Foundation Exams which are related to this scheme. For more detailed information about the Foundation Exams and the overall Master Requirements, please go to the following link:
https://pecb.com/en/master-credentials.
General Information
- Certification and examination fees are included in the price of the training course
- Training material containing over 450 pages of information and practical examples will be distributed
- A participation certificate of 31 CPD (Continuing Professional Development) credits will be issued
- In case of exam failure, you can retake the exam within 12 months for free
- For more information about exam details, please visit Examination Rules and Policies.

